In general, only the first electron affinity can be measured directly, because negative ions repel electrons. However, the second (and third, if necessary) electron affinity can be estimated using a variation on the Born-Haber cycle. Here are the reactions that must be considered if you need to make oxide ions.
Porous Semiconducting K–Sn–Mo–S Aerogel: Synthesis, Local Structure, and Ion-Exchange Properties | Chemistry of Materials
Concept explainers Question Which reaction below represents the electron affinity of K? Expert Solution Trending now This is a popular solution! Step by step Solved in 2 steps See solution Check out a sample Q&A here Knowledge Booster Learn more about Theories of Bonding Need a deep-dive on the concept behind this application? Look no further.

Source Image: sciencedirect.com
Download Image
Ionization energies measure the tendency of a neutral atom to resist the loss of electrons. It takes a considerable amount of energy, for example, to remove an electron from a neutral fluorine atom to form a positively charged ion. = 1681.0 kJ/mol. electron affinity of an element is the energy given off when a neutral atom in the gas phase
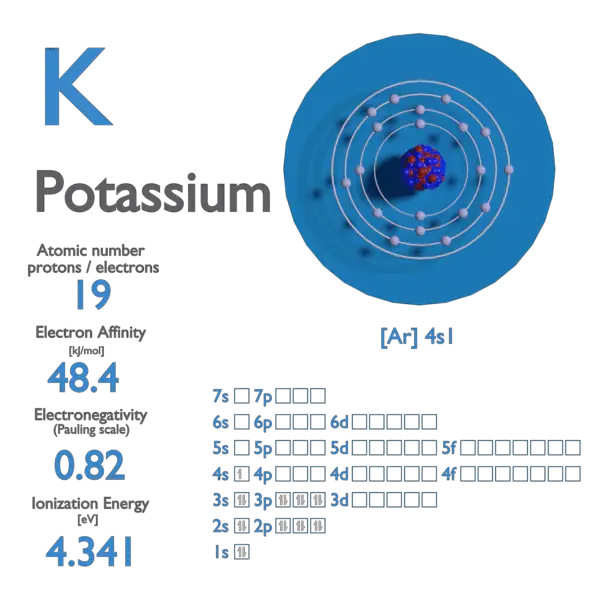
Source Image: nuclear-power.com
Download Image
SOLVED: Which reaction below represents the electron affinity of K? K+(g) + e- → K(g) K(g) + e- → K-(g) K+(g) → K(g) + e- K(g) → K+(g) + e- K(g) + The electron affinity (E ea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion.. X(g) + e − → X − (g) + energy. This differs by sign from the energy change of electron capture ionization. The electron affinity is positive when energy is released on electron capture.
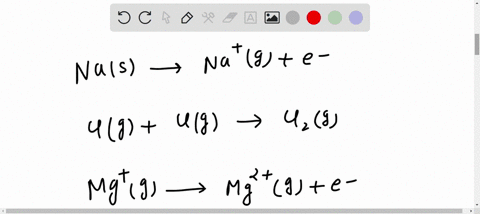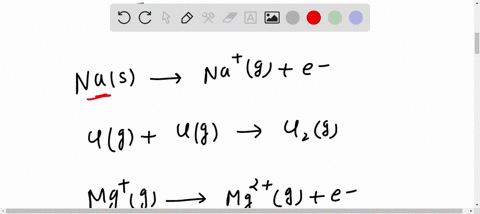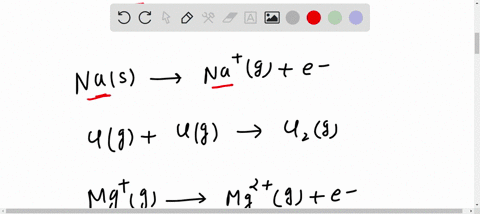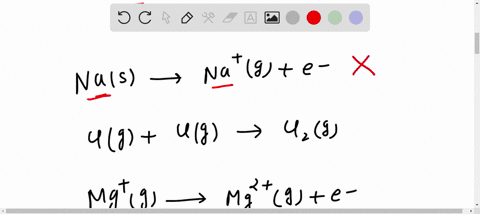
Source Image: numerade.com
Download Image
Which Reaction Below Represents The Electron Affinity Of K
The electron affinity (E ea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion.. X(g) + e − → X − (g) + energy. This differs by sign from the energy change of electron capture ionization. The electron affinity is positive when energy is released on electron capture. An atom of Potassium in the gas phase, for example, gives off energy when it gains an electron to form an ion of Potassium. K + e – → K – – ∆H = Affinity = 48.4 kJ/mol. Electron affinity is one of the most important parameters that guide chemical reactivity.
SOLVED: Which reaction below represents the electron affinity of K? K(g) + e- = K-(g) K(g) = K-(g) + e- K(g) + e- = K-(g) K-(g) = K(g) – e- K(g) –
The electron affinity of potassium (K) can be illustrated by the following chemical reaction: K(g) + e – → K – (g) SOLVED: Which reaction below represents the electron affinity of K? K+(g) + e- → K(g) K(g) + e- → K-(g) K+(g) → K(g) + e- K(g) → K+(g) + e- K(g) +

Source Image: numerade.com
Download Image
Solved QUESTION 9 Which reaction below represents the | Chegg.com The electron affinity of potassium (K) can be illustrated by the following chemical reaction: K(g) + e – → K – (g)

Source Image: chegg.com
Download Image
Porous Semiconducting K–Sn–Mo–S Aerogel: Synthesis, Local Structure, and Ion-Exchange Properties | Chemistry of Materials In general, only the first electron affinity can be measured directly, because negative ions repel electrons. However, the second (and third, if necessary) electron affinity can be estimated using a variation on the Born-Haber cycle. Here are the reactions that must be considered if you need to make oxide ions.

Source Image: pubs.acs.org
Download Image
SOLVED: Which reaction below represents the electron affinity of K? K+(g) + e- → K(g) K(g) + e- → K-(g) K+(g) → K(g) + e- K(g) → K+(g) + e- K(g) + Ionization energies measure the tendency of a neutral atom to resist the loss of electrons. It takes a considerable amount of energy, for example, to remove an electron from a neutral fluorine atom to form a positively charged ion. = 1681.0 kJ/mol. electron affinity of an element is the energy given off when a neutral atom in the gas phase

Source Image: numerade.com
Download Image
Solved 14) Which reaction below represents the electron | Chegg.com Electron Affinity. The electron affinity (EA) of an element E is defined as the energy change that occurs when an electron is added to a gaseous atom or ion: [latex]E_(g)+e^- \rightarrow E^-_(g) \;\;\; \textenergy change=EA \label7.5.1[/latex] Unlike ionization energies, which are always positive for a neutral atom because energy is required to remove an electron, electron affinities

Source Image: chegg.com
Download Image
Lattice Energy Questions – Practice Questions of Lattice Energy with Answer & Explanations The electron affinity (E ea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion.. X(g) + e − → X − (g) + energy. This differs by sign from the energy change of electron capture ionization. The electron affinity is positive when energy is released on electron capture.

Source Image: byjus.com
Download Image
Valence Electrons | Definition, Role & Examples – Video & Lesson Transcript | Study.com An atom of Potassium in the gas phase, for example, gives off energy when it gains an electron to form an ion of Potassium. K + e – → K – – ∆H = Affinity = 48.4 kJ/mol. Electron affinity is one of the most important parameters that guide chemical reactivity.

Source Image: study.com
Download Image
Solved QUESTION 9 Which reaction below represents the | Chegg.com
Valence Electrons | Definition, Role & Examples – Video & Lesson Transcript | Study.com Concept explainers Question Which reaction below represents the electron affinity of K? Expert Solution Trending now This is a popular solution! Step by step Solved in 2 steps See solution Check out a sample Q&A here Knowledge Booster Learn more about Theories of Bonding Need a deep-dive on the concept behind this application? Look no further.
SOLVED: Which reaction below represents the electron affinity of K? K+(g) + e- → K(g) K(g) + e- → K-(g) K+(g) → K(g) + e- K(g) → K+(g) + e- K(g) + Lattice Energy Questions – Practice Questions of Lattice Energy with Answer & Explanations Electron Affinity. The electron affinity (EA) of an element E is defined as the energy change that occurs when an electron is added to a gaseous atom or ion: [latex]E_(g)+e^- \rightarrow E^-_(g) \;\;\; \textenergy change=EA \label7.5.1[/latex] Unlike ionization energies, which are always positive for a neutral atom because energy is required to remove an electron, electron affinities