Because fluoride is the least stable (most basic) of the halide conjugate bases, HF is the least acidic of the haloacids, only slightly stronger than acetic acid. HI, with a pK a of about -9, is one the strongest acids known. More importantly to the study of biological organic chemistry, this trend tells us that thiols are more acidic than
Draw the resonance structure indicated by the arrows. – ppt video online download
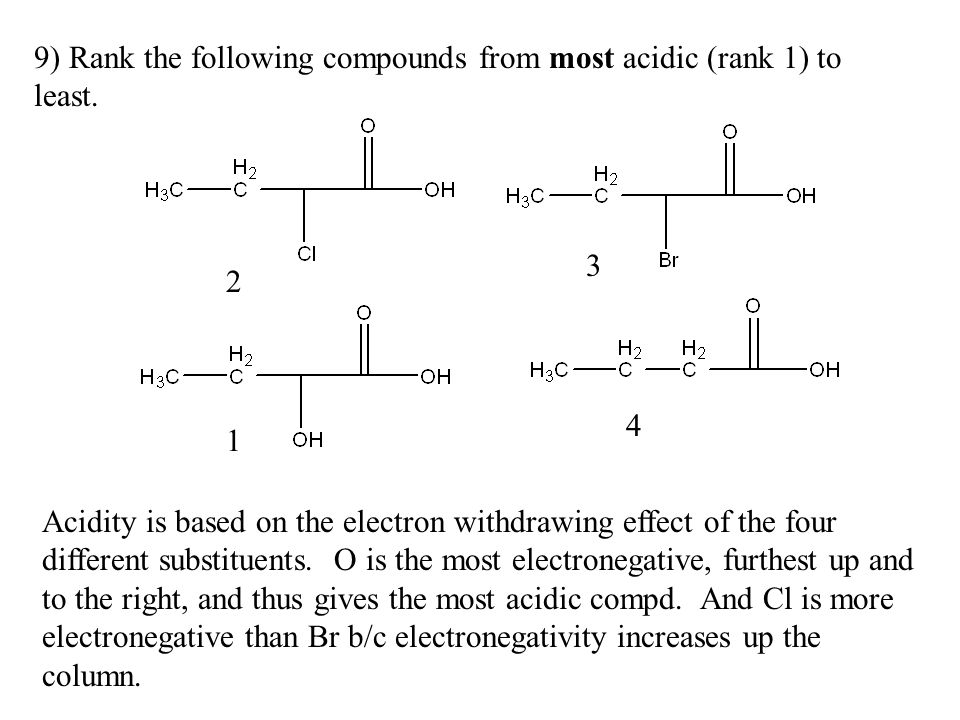
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. II > V > III > I > IV. Expansion of the valence shell to accommodate more than eight electrons is possible with: sulfur. Which of the following species is/are resonance form(s) of the anionic species in the box
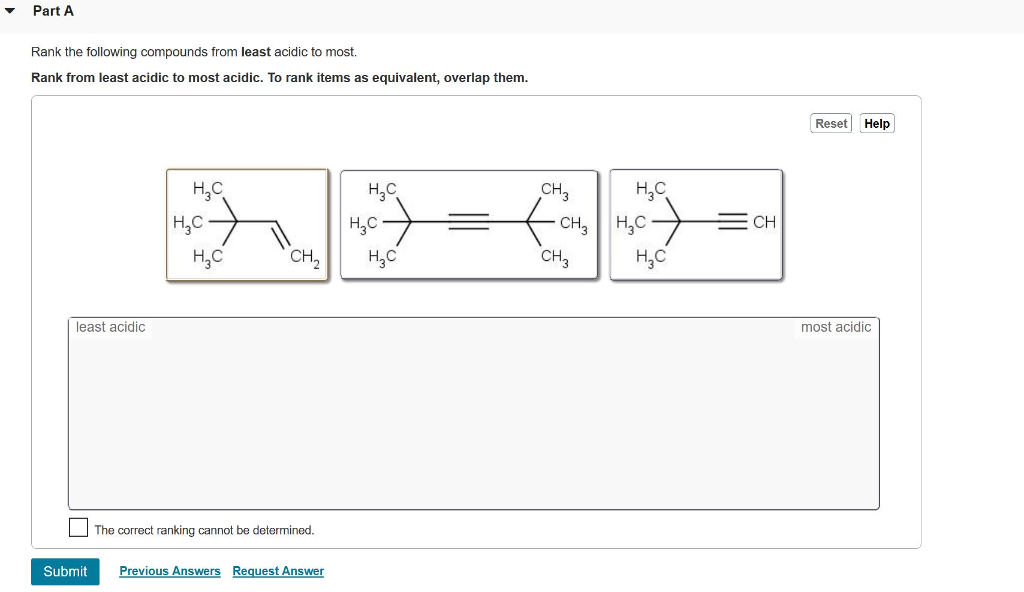
Source Image: www.chegg.com
Download Image
For now, the concept is applied only to the influence of atomic radius on anion stability. Because fluoride is the least stable (most basic) of the halide conjugate bases, HF is the least acidic of the haloacids, only slightly stronger than acetic acid. HI, with a pK a of about -9, is one the strongest acids known.
Source Image: www.chegg.com
Download Image
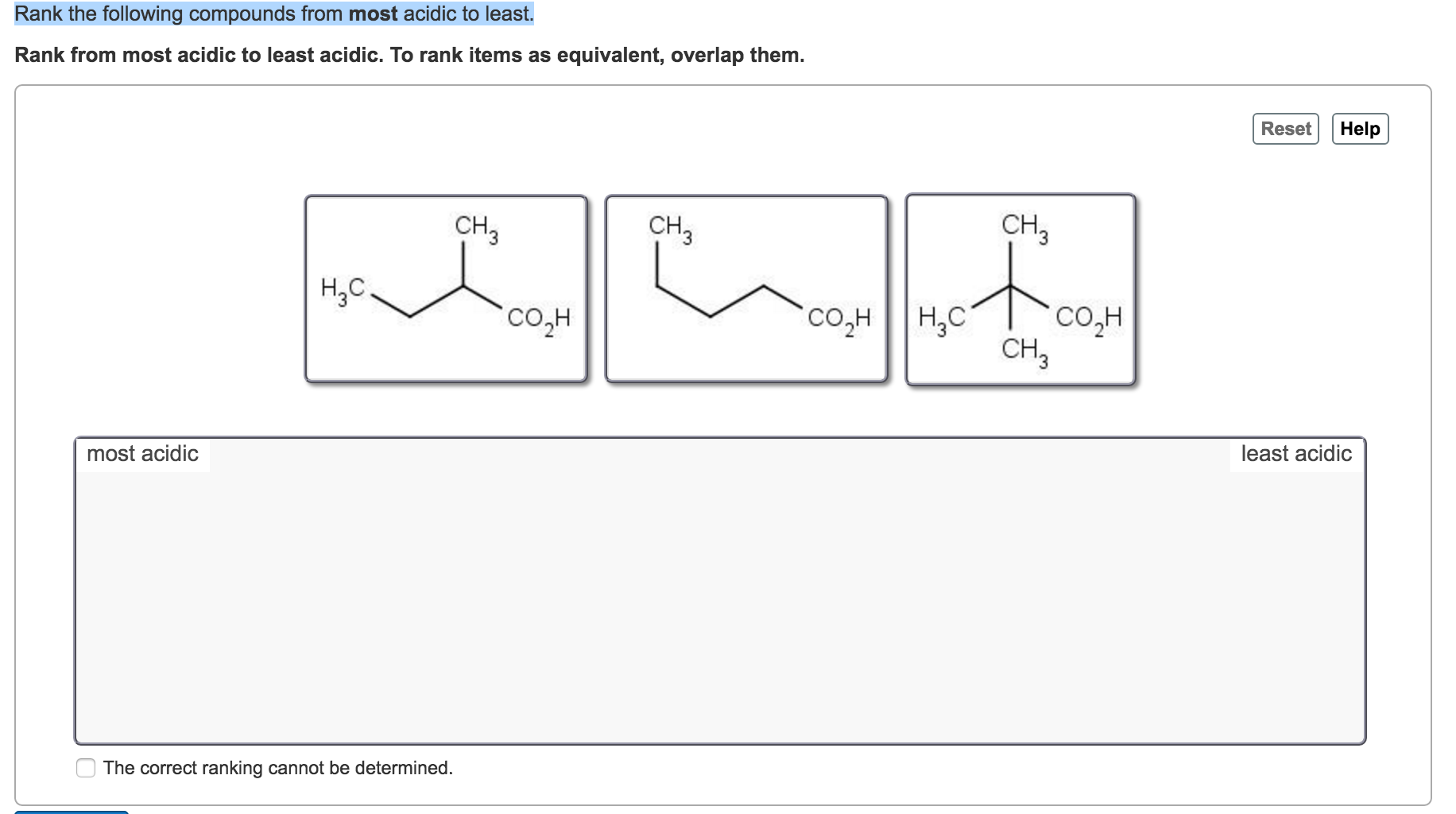
Rank the following compounds in order of decreasing acid strength (most acidit `rarr` least acidic ) – YouTube 40. Question 2n. Textbook Question. The following compounds are listed in increasing order of acidity. In each case, the most acidic proton is shown in red. W pKa=25 X pKa= 23Y pKa= 8.8 Z pKa= 4.2 b. Explain why X is a stronger acid than W. c. Explain why Y is a stronger acid than X. d. Explain why Z is a stronger acid than Y.
Source Image: www.chegg.com
Download Image
Rank The Following Compounds From Most Acidic To Least Acidic
40. Question 2n. Textbook Question. The following compounds are listed in increasing order of acidity. In each case, the most acidic proton is shown in red. W pKa=25 X pKa= 23Y pKa= 8.8 Z pKa= 4.2 b. Explain why X is a stronger acid than W. c. Explain why Y is a stronger acid than X. d. Explain why Z is a stronger acid than Y. Because fluoride is the least stable (most basic) of the halide conjugate bases, HF is the least acidic of the haloacids, only slightly stronger than a carboxylic acid. HI, with a pK a of about -9, is almost as strong as sulfuric acid.
Solved Rank the following compounds from most acidic to | Chegg.com
8 PRACTICE PROBLEM There are four compounds given in increasing order of acidity. The most acidic hydrogen in each of these structures is circled. Explain why each of these compounds is more acidic than the preceding one. 9 PRACTICE PROBLEM Explain the relative acidities of the following conjugate acids. 10 PRACTICE PROBLEM High Acidity Coffee | Volcanica Coffee

Source Image: volcanicacoffee.com
Download Image
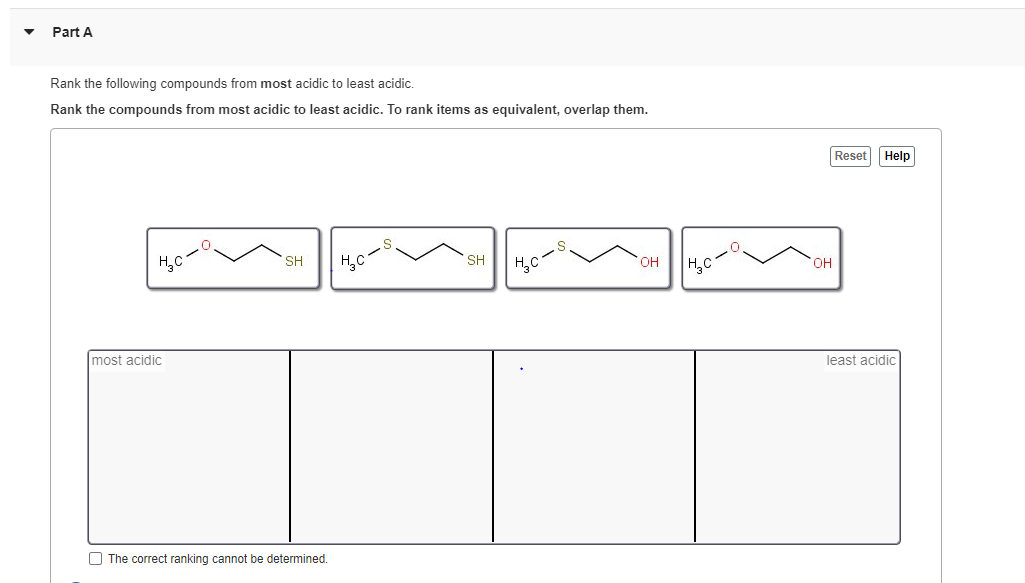
Rank these compounds from the least acidic to the most acidic.FCH… | Channels for Pearson+ 8 PRACTICE PROBLEM There are four compounds given in increasing order of acidity. The most acidic hydrogen in each of these structures is circled. Explain why each of these compounds is more acidic than the preceding one. 9 PRACTICE PROBLEM Explain the relative acidities of the following conjugate acids. 10 PRACTICE PROBLEM

Source Image: www.pearson.com
Download Image
Draw the resonance structure indicated by the arrows. – ppt video online download Because fluoride is the least stable (most basic) of the halide conjugate bases, HF is the least acidic of the haloacids, only slightly stronger than acetic acid. HI, with a pK a of about -9, is one the strongest acids known. More importantly to the study of biological organic chemistry, this trend tells us that thiols are more acidic than
Source Image: slideplayer.com
Download Image
Rank the following compounds in order of decreasing acid strength (most acidit `rarr` least acidic ) – YouTube For now, the concept is applied only to the influence of atomic radius on anion stability. Because fluoride is the least stable (most basic) of the halide conjugate bases, HF is the least acidic of the haloacids, only slightly stronger than acetic acid. HI, with a pK a of about -9, is one the strongest acids known.

Source Image: www.youtube.com
Download Image
Among the following compounds, the most acidic is: – Sarthaks eConnect | Largest Online Education Community Because fluoride is the least stable (most basic) of the halide conjugate bases, HF is the least acidic of the haloacids, only slightly stronger than acetic acid. HI, with a pK a of about -9, is one the strongest acids known. More importantly to the study of biological organic chemistry, this trend tells us that thiols are more acidic than

Source Image: www.sarthaks.com
Download Image
Solved Rank the following compounds from most acidic to | Chegg.com 40. Question 2n. Textbook Question. The following compounds are listed in increasing order of acidity. In each case, the most acidic proton is shown in red. W pKa=25 X pKa= 23Y pKa= 8.8 Z pKa= 4.2 b. Explain why X is a stronger acid than W. c. Explain why Y is a stronger acid than X. d. Explain why Z is a stronger acid than Y.
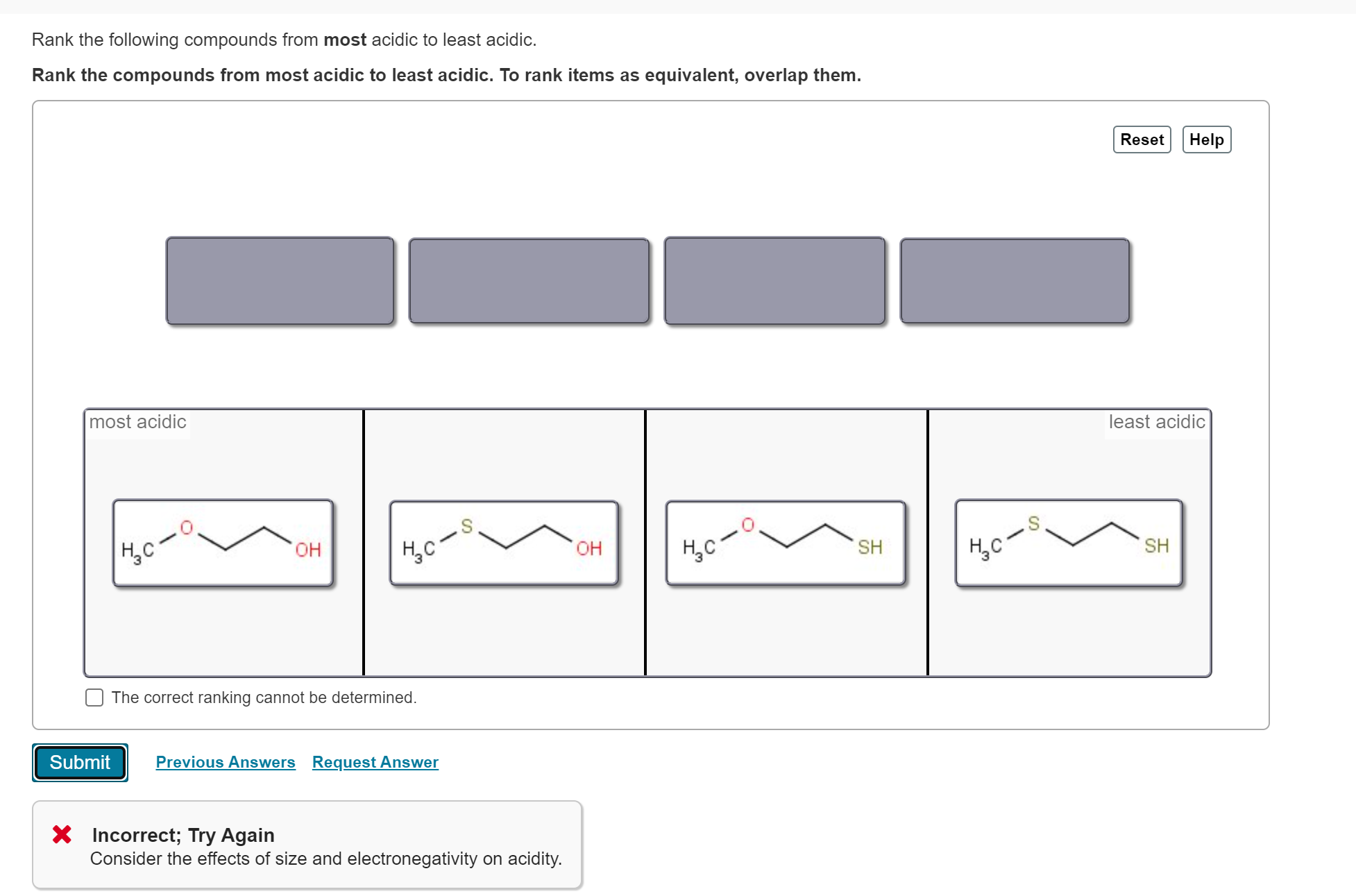
Source Image: www.chegg.com
Download Image
pH Scale Quiz Questions And Answers – Trivia & Questions Because fluoride is the least stable (most basic) of the halide conjugate bases, HF is the least acidic of the haloacids, only slightly stronger than a carboxylic acid. HI, with a pK a of about -9, is almost as strong as sulfuric acid.
.jpg)
Source Image: www.proprofs.com
Download Image
Rank these compounds from the least acidic to the most acidic.FCH… | Channels for Pearson+
pH Scale Quiz Questions And Answers – Trivia & Questions Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. II > V > III > I > IV. Expansion of the valence shell to accommodate more than eight electrons is possible with: sulfur. Which of the following species is/are resonance form(s) of the anionic species in the box
Rank the following compounds in order of decreasing acid strength (most acidit `rarr` least acidic ) – YouTube Solved Rank the following compounds from most acidic to | Chegg.com Because fluoride is the least stable (most basic) of the halide conjugate bases, HF is the least acidic of the haloacids, only slightly stronger than acetic acid. HI, with a pK a of about -9, is one the strongest acids known. More importantly to the study of biological organic chemistry, this trend tells us that thiols are more acidic than